Benzene + 1-chlorobutane And Alcl3
Halogenation of Benzene and Methylbenzene
- Folio ID
- 3978
This page looks at the reactions of benzene and methylbenzene (toluene) with chlorine and bromine nether various atmospheric condition.
The halogenation of benzene
Commutation reactions
Benzene reacts with chlorine or bromine in the presence of a catalyst, replacing one of the hydrogen atoms on the ring by a chlorine or bromine cantlet. The reactions happen at room temperature. The catalyst is either aluminum chloride (or aluminum bromide if you are reacting benzene with bromine) or fe.
Strictly speaking iron is non a catalyst, considering it gets permanently changed during the reaction. It reacts with some of the chlorine or bromine to form iron(III) chloride, \(FeCl_3\), or atomic number 26(III) bromide, \(FeBr_3\).
\[ 2Fe + 3Cl_2 \rightarrow 2FeCl_3\]
\[ 2Fe + 3Br_2 \rightarrow 2FeBr_3\]
These compounds act as the catalyst and behave exactly like aluminum chloride, \(AlCl_3\), or aluminum bromide, \(AlBr_3\), in these reactions.
The reaction with chlorine
The reaction between benzene and chlorine in the presence of either aluminum chloride or iron gives chlorobenzene.
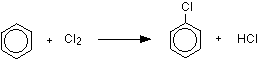
or, written more than compactly:
\[ C_6H_6 + Cl_2 \rightarrow C_6H_5Cl + HCl\]
The reaction with bromine
The reaction betwixt benzene and bromine in the presence of either aluminum bromide or iron gives bromobenzene. Fe is usually used because it is cheaper and is more readily available.
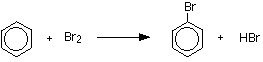
or
\[C_6H_6 + Br_2 \rightarrow C_6H_5Br + HBr\]
Addition reactions
In the presence of ultraviolet low-cal (but without a catalyst present), hot benzene will also undergo an addition reaction with chlorine or bromine. The ring delocalization is permanently broken and a chlorine or bromine atom adds on to each carbon atom.
For instance, if you bubble chlorine gas through hot benzene exposed to UV light for an hour, yous become 1,2,three,4,5,six-hexachlorocyclohexane.
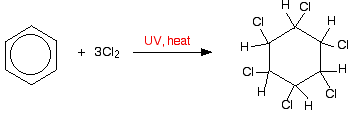
Bromine would behave similarly.
The chlorines and hydrogens can stick up and down at random above and below the band and this leads to a number of geometric isomers. Although there aren't any carbon-carbon double bonds, the bonds are still "locked" and unable to rotate. One of these isomers was once normally used as an insecticide known variously as BHC, HCH and Gammexane. This is one of the "chlorinated hydrocarbons" which caused so much environmental damage.
The halogenation of methylbenzene
Substitution reactions
It is possible to get two quite unlike substitution reactions between methylbenzene and chlorine or bromine depending on the weather condition used. The chlorine or bromine tin substitute into the ring or into the methyl grouping.
Substitution into the band
Substitution in the ring happens in the presence of aluminum chloride (or aluminum bromide if you are using bromine) or iron, and in the absenteeism of UV light. The reactions happen at room temperature. This is exactly the aforementioned as the reaction with benzene, except that yous take to worry about where the halogen atom attaches to the ring relative to the position of the methyl group.
Methyl groups are two,iv-directing, which means that incoming groups will tend to go into the ii or 4 positions on the ring - assuming the methyl group is in the 1 position. In other words, the new group volition attach to the ring side by side door to the methyl group or opposite information technology. With chlorine, substitution into the ring gives a mixture of ii-chloromethylbenzene and 4-chloromethylbenzene.
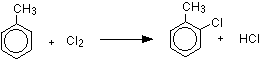
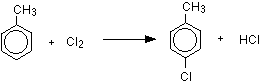
With bromine, you would go the equivalent bromine compounds.
Substitution into the methyl group
If chlorine or bromine react with boiling methylbenzene in the absenteeism of a goad simply in the presence of UV light, substitution happens in the methyl grouping rather than the band. For instance, with chlorine (bromine would be similar):
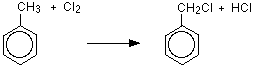
The organic product is (chloromethyl)benzene. The brackets in the proper noun emphasize that the chlorine is part of the fastened methyl group, and isn't on the ring.
One of the hydrogen atoms in the methyl group has been replaced past a chlorine atom. Even so, the reaction doesn't stop there, and all three hydrogens in the methyl group can in turn exist replaced by chlorine atoms. That means that you could likewise get (dichloromethyl)benzene and (trichloromethyl)benzene as the other hydrogen atoms in the methyl grouping are replaced one at a fourth dimension.
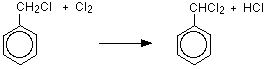
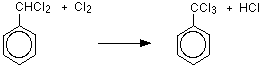
If y'all use plenty chlorine yous will eventually get (trichloromethyl)benzene, only any other proportions will always atomic number 82 to a mixture of products.
Addition reactions
I haven't been able to track down anything like to the reaction between benzene and chlorine in which vi chlorine atoms add around the band.
That possibly isn't surprising. Chlorine adds to benzene in the presence of ultraviolet light. With methylbenzene under those conditions, you lot become substitution in the methyl group. That is energetically easier because it doesn't involve breaking the delocalized electron arrangement.
Whether you would get addition to the ring if you used a big backlog of chlorine and did the reaction for a long time, I don't know. Once all the hydrogens in the methyl group had been substituted, perhaps y'all might and so get addition to the band as well.
Benzene + 1-chlorobutane And Alcl3,
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Arenes/Reactivity_of_Arenes/Halogenation_of_Benzene_and_Methylbenzene
Posted by: steinmetzocas1943.blogspot.com


0 Response to "Benzene + 1-chlorobutane And Alcl3"
Post a Comment